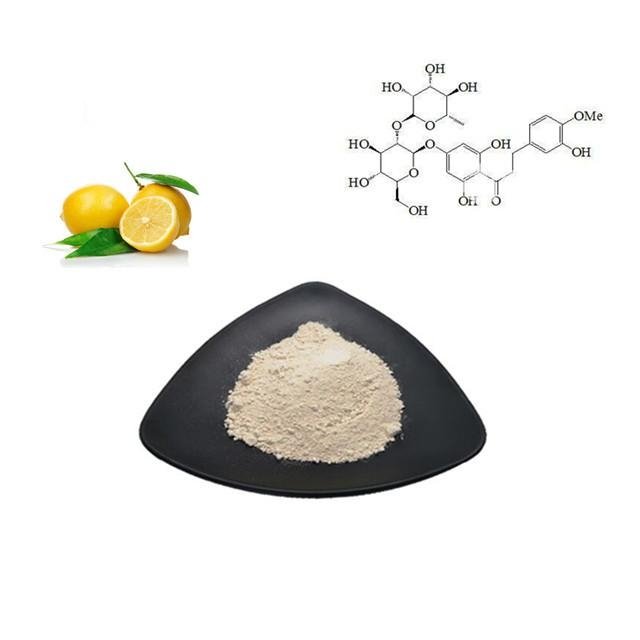
Neohesperidin dihydrochalcone
1.Name:Neohesperidin dihydrochalcone / NHDC
2.Appearance:White powder
3.Specs.: 98%
4.CAS. 20702-77-6
5.Test method: HPLC
6.Active ingredient: Neohesperidin dihydrochalcone
7. Appearance: White powder

What is Neohesperidin?
Neohesperidin dihydrochalcone(NHDC), was discovered during the 1960s for minimizing the taste of bitter flavorants in citrus juices. Neohesperidin when treated with potassium hydroxide or another strong base, and then catalytically hydrogenated, it becomes NHDC, a compound roughly 1500-1800 times sweeter than sugar at threshold concentrations; around 340 times sweeter than sugar weight-for-weight.
Like other highly sweet glycosides, such as glycyrrhizin and those found in stevia, NHDC's sweet taste has a slower onset than sugar's and lingers in the mouth for some time. Unlike aspartame, NHDC is stable to elevated temperatures and to acidic or basic conditions, and so can be used in applications that require a long shelf life. NHDC itself can stay foodsafe for up to five years when stored in optimal conditions.
NHDC in pure form is found as a white substance not unlike powdered sugar. In food it is used as a flavour enhancer in concentrations of around 4-5 parts per million (ppm) and as an artificial sweetener at around 15-20 ppm.
| Citrus aurantium extract |
||||
| Active Ingredients |
Specs |
Test Method |
Appearance |
Solubility |
| Synephrine |
6%-30% |
HPLC |
Brown-yellow powder |
Slightly soluble in water and methanol |
| Hesperidin |
10%-98% |
HPLC |
Yellow to light brown powder |
Slightly soluble in methanol |
| Hesperetin |
10%-98% |
HPLC |
Yellow to dark brown powder |
Slightly soluble in water and methanol |
| Neohesperidin |
10%-98% |
HPLC |
Off-White or light yellow powder |
soluble in hot water and ethanol |
| Diosmin/ Hesperidin |
9:1 |
HPLC |
Grayish Yellow or yellow powder |
Slightly soluble in water |
| Citrus Bioflavonoids |
10%-90% |
HPLC |
Brown-yellow powder |
Slightly soluble in water |
| Nobiletin |
98% |
HPLC |
White Crystalline powder |
soluble in hot water and ethanol |
| PMFs (Polymethoxy Flavones) |
10%-98% |
HPLC |
Yellow to Brown |
Partially soluble in hot water and ethanol |
| NHDC |
98% |
HPLC |
White |
soluble in water |
| Citrus Polyphenols |
10%-90% |
HPLC |
Yellow to Brown |
soluble in water |
NHDC main function:
1. Enhance the action of Vitamin C: relief the blood cell coagulation in conjunctiva of guinea pig due to lack of Vitamin C; it is also reported that it can reduce blood cell coagulation in horse. The life span of tats is prolonged when the product is fed with thrombogenic feed or feed that may cause atherosis. Can raise the Vitamin C concentration in adrenal gland, spleen and white blood cell in guinea pig.
2. All capability: when fibrocytes of mice are treated with the product in 200μg/ml solution, the cells can resist the attack from phlyctenular stomatitis virus for 24 hours. Hela cells treated with the product can resist the infection from flu virus. The antiviral activity of the product may be attenuated by hyaluronidase.
3. Other: prevent injury from cold; inhibit aldehyde reductase in lens of rat eyes
NHDC application
1. Applied in food field, it is used as a flavor enhancer and sweetener in a wide variety of Alcoholic beverage, dessert foods and savory foods.
2. Applied in cosmetics, it can added into toothpaste and mouth wash.
3. Applied in pharmaceutical field, it is mainly used to reducing the bitterness of in form.
4. Applied in Feed Field,Used for livestock feed as a means of reducing feeding time.
For more product information pls kindly contact email sales09@staherb.cn
Physical index
| Botanical Source: |
Citrus Aurantium L |
| Part used: |
Fruit |
| Specification: |
NHDC 98% |
| Appearance |
White fine powder |
| Flavor & Odor |
Characteristic |
| Particle size |
100% pass 80 mesh |
| Physical: |
|
| Loss on Drying |
≤1.0% |
| Bulk density |
40-60g/100ml |
| Sulphated Ash |
≤1.0% |
| GMO |
Free |
| General Status |
Non-irradiated |
| Chemical: |
|
| Pb |
≤2mg/kg |
| As |
≤1mg/kg |
| Hg |
≤0.1mg/kg |
| Cd |
≤1.0mg/kg |
| Microbial: |
|
| Total microbacterial count |
≤1000cfu/g |
| Yeast & Mold |
≤100cfu/g |
| E.Coli |
Negative |
| Staphylococcus aureus |
Negative |
| Salmonella |
Negative |
| Enterobacteriaceaes |
Negative |
References:
- 1.
Montmayeur JP, Liberles SD, Matsunami H, Buck LB: A candidate taste receptor gene near a sweet taste locus. Nat Neurosci 2001, 4: 492–498.
- 2.
Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF: Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 2003, 301: 850–853. 10.1126/science.1087155
- 3.
Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS: Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell 1999, 96: 541–551. 10.1016/S0092-8674(00)80658-3
- 4.
Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E: Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A 2002, 99: 4692–4696. 10.1073/pnas.072090199
- 5.
Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS: Mammalian sweet taste receptors. Cell 2001, 106: 381–390. 10.1016/S0092-8674(01)00451-2
- 6.
Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF: Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet 2001, 28: 58–63.
- 7.
Terrillon S, Bouvier M: Roles of G-protein-coupled receptor dimerization. EMBO Rep 2004, 5: 30–34.
- 8.
Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS: An amino-acid taste receptor. Nature 2002, 416: 199–202. 10.1038/nature726
- 9.
Brouwer JN, Hellekant G, Kasahara Y, van der Wel H, Zotterman Y: Electrophysiological study of the gustatory effects of the sweet proteins monellin and thaumatin in monkey, guinea pig and rat. Acta Physiol Scand 1973, 89: 550–557.
- 10.
Sclafani A, Abrams M: Rats show only a weak preference for the artificial sweetener aspartame. Physiol Behav 1986, 37: 253–256. 10.1016/0031-9384(86)90228-3
- 11.
Sclafani A, Perez C: Cypha [propionic acid, 2-(4-methoxyphenol) salt] inhibits sweet taste in humans, but not in rats. Physiol Behav 1997, 61: 25–29. 10.1016/S0031-9384(96)00316-2
- 12.
Jiang P, Ji Q, Liu Z, Snyder LA, Benard LM, Margolskee RF, Max M: The cysteine-rich region of T1R3 determines responses to intensely sweet proteins. J Biol Chem 2004, 279: 45068–45075.
- 13.
Xu H, Staszewski L, Tang H, Adler E, Zoller M, Li X: Different functional roles of T1R subunits in the heteromeric taste receptors. Proc Natl Acad Sci U S A 2004, 101: 14258–14263.
- 14.
Jiang P, Cui M, Zhao B, Snyder LA, Benard LM, Osman R, Max M, Margolskee RF: Identification of the cyclamate interaction site within the transmembrane domain of the human sweet taste receptor subunit T1R3. J Biol Chem 2005, 280: 34296–34305.
- 15.
Jiang P, Cui M, Zhao B, Liu Z, Snyder LA, Benard LM, Osman R, Margolskee RF, Max M: Lactisole interacts with the transmembrane domains of human T1R3 to inhibit sweet taste. J Biol Chem 2005, 280: 15238–15246.
- 16.
Winnig M, Bufe B, Meyerhof W: Valine 738 and lysine 735 in the fifth transmembrane domain of rTas1r3 mediate insensitivity towards lactisole of the rat sweet taste receptor. BMC Neurosci 2005,
- 17.
Dogan M: Neohesperidin DC in food products: ; Trabzon, Türkiye. Volume 1. ; 2002:190–195.
- 18.
DuBois GE, Crosby GA, Stephenson RA, Wingard RE Jr.: Dihydrochalcone sweeteners. Synthesis and sensory evaluation of sulfonate derivatives. J Agric Food Chem 1977, 25: 763–772.
- 19.
DuBois GE, Crosby GA, Stephenson RA: Dihydrochalcone sweeteners. A study of the atypical temporal phenomena. J Med Chem 1981, 24: 408–428.
- 20.
Durroux T: Principles: a model for the allosteric interactions between ligand binding sites within a dimeric GPCR. Trends Pharmacol Sci 2005, 26: 376–384. 10.1016
- 21.
Morini G, Bassoli A, Temussi PA: From small sweeteners to sweet proteins: anatomy of the binding sites of the human T1R2_T1R3 receptor. J Med Chem 2005, 48: 5520–5529.
- 22.
Kratochwil NA, Malherbe P, Lindemann L, Ebeling M, Hoener MC, Muhlemann A, Porter RH, Stahl M, Gerber PR: An automated system for the analysis of G protein-coupled receptor transmembrane binding pockets: alignment, receptor-based pharmacophores, and their application. J Chem Inf Model 2005
- 23.
Whitelaw ML, Chung HJ, Daniel JR: Synthesis and sensory evaluation of ring-substituted dihydrochalcone sweeteners. 2. Analogues of 3'-Carboxyhesperetin dihydrocahlcone, a high-potency dihydrochalcone sweetener. J Agric Food Chem 1991, 39: 663–667.
- 24.
Whitelaw ML, Daniel JR: Synthesis and sensory evaluation of ring-substituted dihydrochalcone sweeteners. J Agric Food Chem 1991, 39: 44–51.
- 25.
Naim M, Rogatka H, Yamamoto T, Zehavi U: Taste responses to neohesperidin dihydrochalcone in rats and baboon monkeys. Physiol Behav 1982, 28: 979–986. 10.1016/0031-9384(82)90163-9
- 26.
Bachmanov AA, Tordoff MG, Beauchamp GK: Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses 2001, 26: 905–913. 10.1093
- 27.
Ballesteros JA, H. W: Integrated Methods for the Construction of Three-Dimensional Models and Computational Probing of Structure-Function Relations in G-Protein-Coupled Receptors. Methods in Neuroscience 1995, 25: 366–428.
- 28.
Bassoli A, Merlini L, Morini G: Isovanillyl sweeteners. From molecules to receptors. Pure Appl Chem 2002
- 29.
DuBois GE, Crosby GA, Saffron P: Nonnutritive sweeteners: taste-structure relationships for some new simple dihydrochalcones. Science 1977, 195: 397–399. 10.1126/science.831282
- 30.
Horowitz RM, Gentili B: Flavonoids of the Ponderosa lemon. Nature 1960
- 31.
Schiffman SS, Booth BJ, Sattely-Miller EA, Graham BG, Gibes KM: Selective inhibition of sweetness by the sodium salt of +/-2-(4-methoxyphenoxy)propanoic acid. Chem Senses 1999, 24: 439–447.
- 32.
Hu J, McLarnon SJ, Mora S, Jiang J, Thomas C, Jacobson KA, Spiegel AM: A region in the seven-transmembrane domain of the human Ca2+ receptor critical for response to Ca2+. J Biol Chem 2005
- 33.
Malherbe P, Kratochwil N, Knoflach F, Zenner MT, Kew JN, Kratzeisen C, Maerki HP, Adam G, Mutel V: Mutational analysis and molecular modeling of the allosteric binding site of a novel, selective, noncompetitive antagonist of the metabotropic glutamate 1 receptor. J Biol Chem 2003, 278: 8340–8347. 10.1074/jbc.M211759200
- 34.
Petrel C, Kessler A, Maslah F, Dauban P, Dodd RH, Rognan D, Ruat M: Modeling and mutagenesis of the binding site of Calhex 231, a novel negative allosteric modulator of the extracellular Ca(2+)-sensing receptor. J Biol Chem 2003, 278: 49487–49494. 10.1074/jbc.M308010200
- 35.
Pin JP, Galvez T, Prezeau L: Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol Ther 2003, 98: 325–354. 10.1016/S0163-7258(03)00038-X
- 36.
Swaminath G, Deupi X, Lee TW, Zhu W, Thian FS, Kobilka TS, Kobilka B: Probing the beta2 adrenoceptor binding site with catechol reveals differences in binding and activation by agonists and partial agonists. J Biol Chem 2005, 280: 22165–22171. 10.1074/jbc.M502352200
- 37.
Hu J, Reyes-Cruz G, Chen W, Jacobson KA, Spiegel AM: Identification of acidic residues in the extracellular loops of the seven-transmembrane domain of the human Ca2+ receptor critical for response to Ca2+ and a positive allosteric modulator. J Biol Chem 2002, 277: 46622–46631. 10.1074/jbc.M207100200
- 38.
Malherbe P, Kratochwil N, Zenner MT, Piussi J, Diener C, Kratzeisen C, Fischer C, Porter RH: Mutational analysis and molecular modeling of the binding pocket of the metabotropic glutamate 5 receptor negative modulator 2-methyl-6-(phenylethynyl)-pyridine. Mol Pharmacol 2003, 64: 823–832. 10.1124
- 39.
Miedlich SU, Gama L, Seuwen K, Wolf RM, Breitwieser GE: Homology modeling of the transmembrane domain of the human calcium sensing receptor and localization of an allosteric binding site. J Biol Chem 2004, 279: 7254–7263. 10.1074/jbc.M307191200
- 40.
Pagano A, Ruegg D, Litschig S, Stoehr N, Stierlin C, Heinrich M, Floersheim P, Prezeau L, Carroll F, Pin JP, Cambria A, Vranesic I, Flor PJ, Gasparini F, Kuhn R: The non-competitive antagonists 2-methyl-6-(phenylethynyl)pyridine and 7-hydroxyiminocyclopropan[b]chromen-1a-carboxylic acid ethyl ester interact with overlapping binding pockets in the transmembrane region of group I metabotropic glutamate receptors. J Biol Chem 2000
- 41.
Petrel C, Kessler A, Dauban P, Dodd RH, Rognan D, Ruat M: Positive and negative allosteric modulators of the Ca2+-sensing receptor interact within overlapping but not identical binding sites in the transmembrane domain. J Biol Chem 2004, 279: 18990–18997. 10.1074/jbc.M400724200
- 42.
Ray K, Tisdale J, Dodd RH, Dauban P, Ruat M, Northup JK: Calindol, a positive allosteric modulator of the human Ca(2+) receptor, activates an extracellular ligand-binding domain-deleted rhodopsin-like seven-transmembrane structure in the absence of Ca(2+). J Biol Chem 2005, 280: 37013–37020. 10.1074/jbc.M506681200
- 43.
Schaffhauser H, Rowe BA, Morales S, Chavez-Noriega LE, Yin R, Jachec C, Rao SP, Bain G, Pinkerton AB, Vernier JM, Bristow LJ, Varney MA, Daggett LP: Pharmacological characterization and identification of amino acids involved in the positive modulation of metabotropic glutamate receptor subtype
- 44.
Ueda T, Ugawa S, Yamamura H, Imaizumi Y, Shimada S: Functional interaction between T2R taste receptors and G-protein alpha subunits expressed in taste receptor cells. J Neurosci 2003, 23: 7376–7380.